Things every student should know: the biology and chemistry behind fall foliage
by Ben Trachtman ’12
SCIENCE & TECHNOLOGY EDITOR
Hamilton is one of the most beautiful places in the world in the fall. The leaves begin to change colors, turning whole landscapes brilliant shades of red and yellow. But how and why does this happen? It turns out that one of the most beautiful natural displays in the world is caused by just a few types of miniscule molecules.
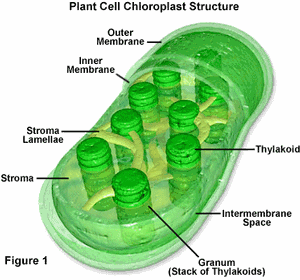
For most the year, leaves are green. Most people know that this color is due to chlorophyll, the green pigment that lets plants convert light into energy. Chlorophyll is contained within chloroplasts, the powerhouses of plant cells, which are concentrated in leaves. When light hits chloroplasts, it sets off a chain of chemical reactions that end with the production of sugar—food for the plant. Because chloroplasts are necessary to provide the plant with energy, they are continually replenished and maintained during the growing season, resulting in green color during the spring and summer.
But in the fall days get shorter and nights get colder as Earth’s tilt exposes the northern hemisphere to less sunlight. Chloroplasts require a great deal of upkeep and do not produce enough energy during the short days to make them worth maintaining. So plants go into the equivalent of hibernation: they shut down most of their metabolic function and live off of food stores accumulated during the summer. The first step in this process is to get rid of their leaves.
As the days get shorter, trees begin building a wall of cells between their branches and leaves. This wall blocks the flow of water and nutrients to and from the leaves, and the leaves begin to die. As chloroplasts die, chlorophyll breaks down, leading to a loss of the characteristic green coloration. This allows other colors that have been present all along but were overwhelmed by the green to shine through.
Yellow leaves are the result of carotenes. The best known example is beta-carotene, which gives carrots their orange color. Other carotenes are even present in the human eye and are involved in light and color detection. Carotenes are involved with photosynthesis and are responsible for a range of colors in nature, from the yellow in daffodils to the red in tomatoes. These molecules are found in tree leaves year-round due to their role in helping plants obtain energy, but are never really seen because the green chlorophyll overpowers them. Without chlorophyll, though, carotenes begin to turn leaves yellow.
Although yellow is a major component of autumn colors, red is often even more potent. The red color in leaves comes from a family of molecules called anthrocyanins. Unlike carotenes, anthrocyanins are not constantly present in leaves, rather they are only synthesized once chloroplasts start to die off. So why should a tree spend energy to produce this dye in leaves it no longer needs? One theory is that the dark red pigment absorbs more light than any of the other colors in the leaf. Light can potentially destabilize molecules, so shielding them with a dark pigment can make them more stable. This might make it easier for the tree to recover as many nutrients from the leaves as it can before it sacrifices them.
Another theory is that the red coloration serves as a warning signal to insects who use the trees as hosts over the winter. Only healthy trees can afford to “waste” the energy to produce this pigment, showing potential parasites that they shouldn’t bother wasting their time feeding off the tree, as its immune system might be able to fend them off. This way, the parasites don’t waste their time, and potentially their lives, trying to live off a tree that can kill them, and the tree is able to get through the winter without any parasites.
While fall foliage is pleasing to the eye, it also serves an important function for the trees themselves. Dropping leaves lets trees get through the winter without wasting nutrients or losing excess water through their leaves.
